Breast and Prostate Cancer: More Similar Than Different
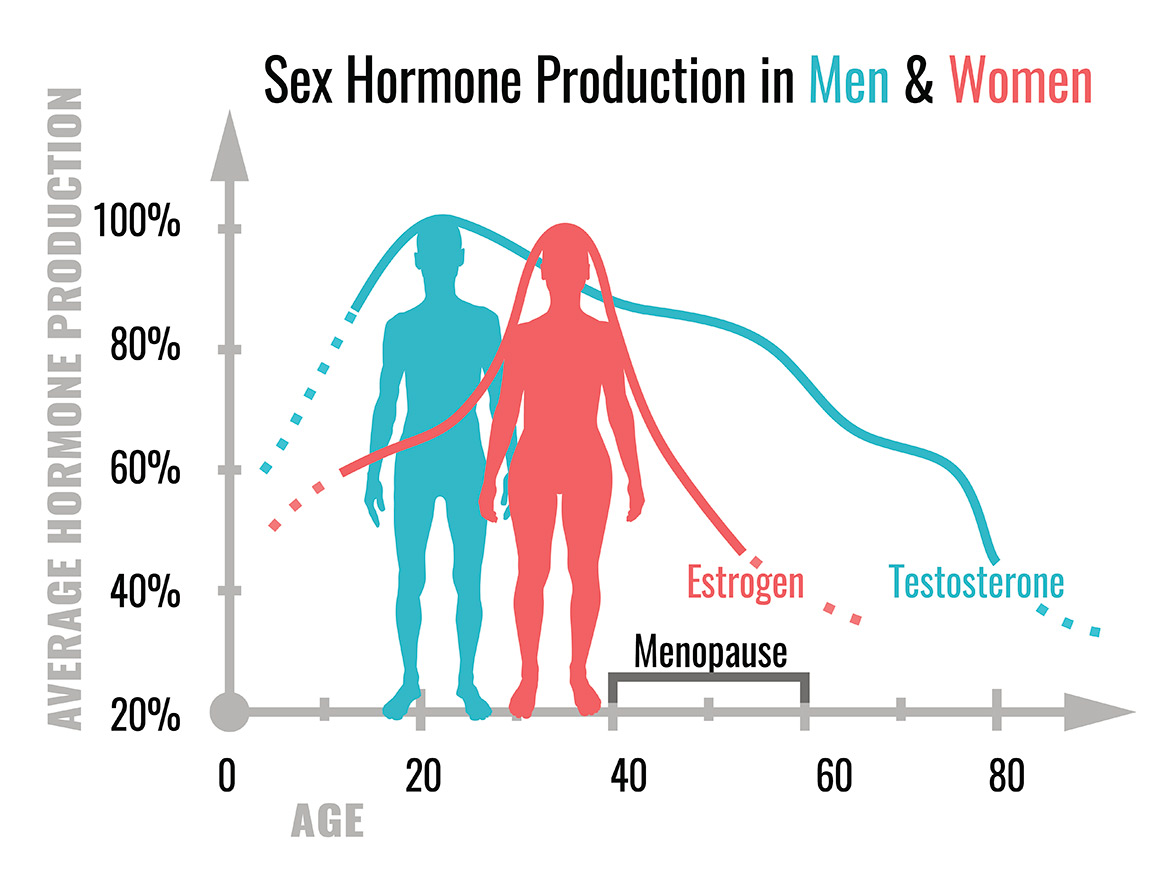
Breast and prostate cancer are the two most common invasive cancers in women and men, respectively. Although these cancers arise in organs of different anatomy and physiological function, both organs are dependent on sex hormone activity (e.g. estrogen and testosterone) for normal development and function.
Interestingly, cancers that arise in the breast and prostate are also typically dependent on sex hormone activity, which is corrupted away from promoting normal tissue function to promoting the growth and spread of cancer cells. Since sex hormones and their corresponding receptors work in similar ways, breast and prostate cancers have remarkable underlying biological similarities that we leverage to maximise the impact of our research.
-
Progression and treatment of breast and prostate cancer
Most breast and prostate cancer patients are cured by local interventions such as surgery and radiation, often in combination with adjuvant endocrine or chemical therapies. However, many patients experience disease recurrence after a number of years (or even decades) and the newly emergent cancers are typically more aggressive, more widely spread (metastatic) and often fatal. The therapeutic strategy employed to treat advanced breast and prostate cancers involves some form of hormonal deprivation to block estrogen receptor (ER) activity in breast cancer and androgen receptor (AR) activity in prostate cancer. Hormone deprivation therapies generally work by blocking the ability to make hormones (estrogen or androgen) or the ability of receptors (ER or AR) to bind hormones.
The knowledge that sex hormones and their receptors drive breast and prostate cancers was a major breakthrough, and the hormone deprivation therapy treatments resulting from this knowledge have saved innumerable lives. Indeed, the hormone deprivation therapy strategy has been the mainstay of treatment for breast and prostate cancers that have spread to other vital organs (i.e. bone, liver, brain) for more than 100 years (breast) or 70 years (prostate). Although initially effective, hormone deprivation therapy is not curative, especially once disease becomes metastatic. After a variable period of response, patients eventually relapse with more lethal forms of disease. Despite new drugs that more effectively inhibit ER or AR, patients still mainly die of ER-driven (breast) or AR-driven (prostate) cancer that becomes resistant to any form of hormone deprivation therapy. Thus, new treatment strategies are required to improve survival rates from these advanced, therapy-resistant stages of disease.
Sex hormone dependent drivers of breast and prostate cancer
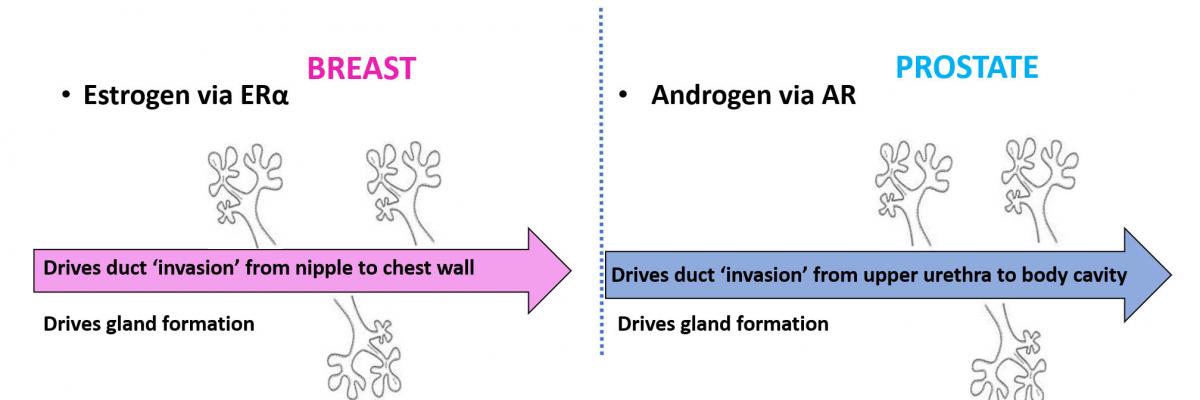
Breast and prostate cancer progression
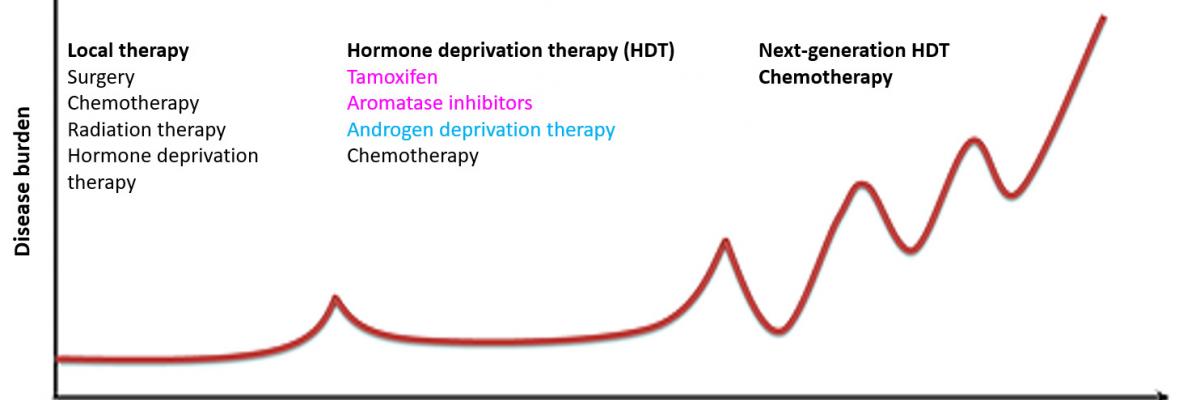
In summary, the current treatment paradigm of hormone deprivation therapy has prolonged life for many women and men with breast or prostate cancer, but….
- Treatment resistance
Most women and men who die of breast or prostate cancer have ER+ or AR+ therapy-resistant disease, often associated with altered ER and AR signalling, and development of aggressive disease. - Treatment side effects
Sex hormone deprivation can make patients feel miserable from joint pain, loss of libido, impairment of cognitive function, early menopause in women and erectile dysfunction in men. Many stop taking the drugs due to these side effects.
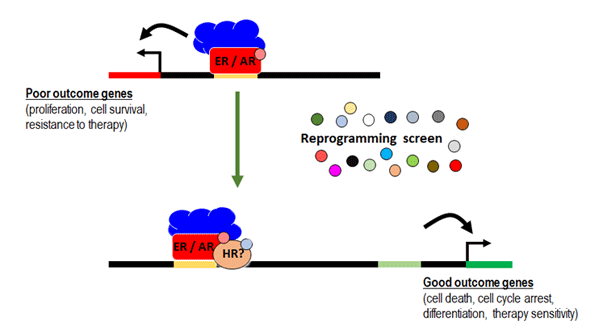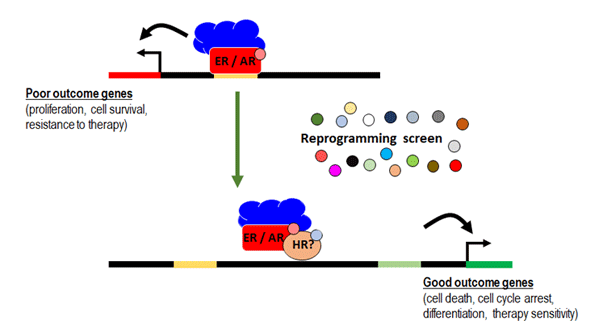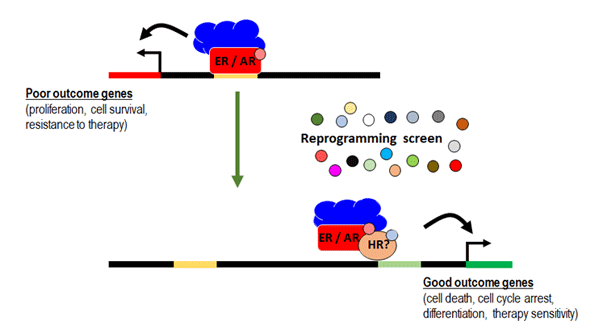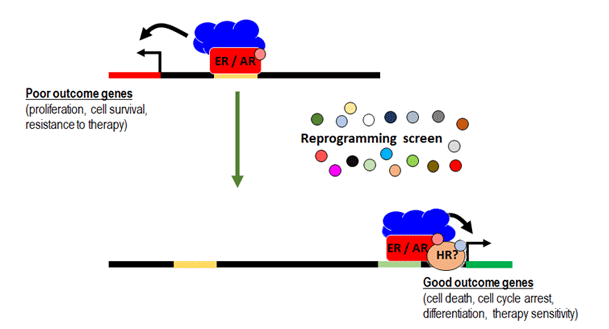
A major focus of our research is to develop a new approach to outsmart these cancers that involves the selective reprogramming of sex hormone receptor activity in breast and prostate cancers rather than wholesale elimination of sex hormone activity within all body tissues.
- Treatment resistance
-
A new treatment paradigm is required
Since breast and prostate cancers are exquisitely dependent on sex hormone activity, hormone deprivation therapies that systemically eliminate this activity from the body impose a pressure toward the evolution of therapy resistant disease. Known mechanisms of therapy resistance include alterations (e.g. mutations) to ER or AR that enable them to drive cancer growth independently of sex hormones. Therefore, we believe a new treatment paradigm is required to establish an alternative to hormone deprivation therapy that does not promote the evolution of receptor mutations and treatment resistance.
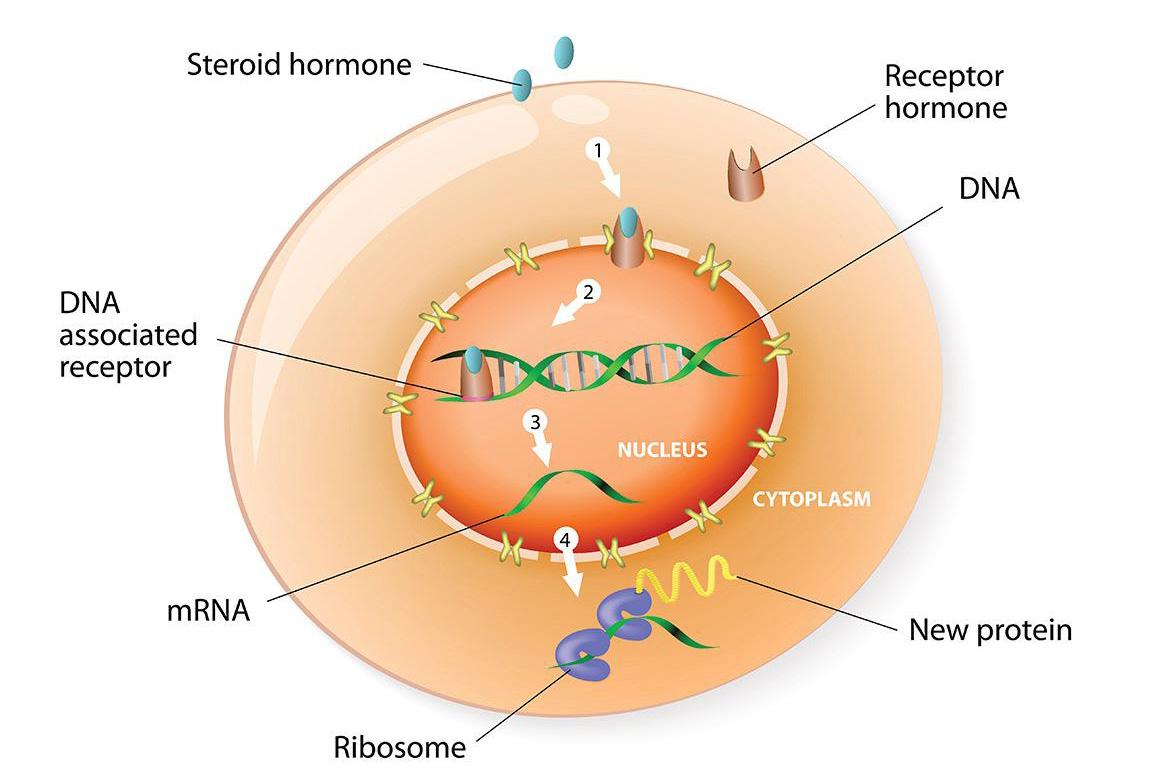
Steroid hormones
Estrogen and androgen sex hormones act by binding to their cognate receptors (i.e. ER or AR), which then act as transcription factors, directly binding to DNA to regulate expression of target genes. Sex hormone receptors belong to a large family of structurally similar transcription factors collectively called nuclear receptors (NRs). NRs are critically important factors that regulate the expression of genes controlling development, homeostasis, and metabolism of an organism. Like ER and AR, other NRs develop corrupted activity associated with cancer and other diseases. Indeed, over 10% of U.S. Food and Drug Administration (FDA) approved drugs target NRs.
Since ER and AR switch from their usual role as 'good players' that promote normal tissue development to 'bad players' that stimulate the growth and spread of cancer cells, we have focused on defining how their patterns of DNA binding differ in normal and cancer tissues at different states of disease. We do this with a view to finding ways to reprogram DNA binding from a disease pattern to a more normal pattern, effectively ‘rehabilitating’ corrupted ER or AR activity. This reprogramming forms the basis for a new paradigm to treat breast and prostate cancers. To do this we are taking a two-pronged approach:
- Investigating repurposed drugs known to be safe and already approved for other medical purposes, as these drugs can be rapidly translated into the clinic;
- Screening thousands of novel NR compounds that may work singly or in combination with existing drugs that have potential for new drug development.
Based on our publications to date, clinical trials are underway to provide proof-of-principle that this reprogramming strategy can work in clinical breast cancers. The benefits and discoveries from this research have strong potential to open new treatment options for breast and prostate cancer, thereby significantly increasing the lifespan of patients. These drugs also have potential to improve quality of life for patients with hormone-sensitive breast and prostate cancers, and to be used in other types of cancers in the future.
-
Our research approaches
Researchers, collaborators and clinical affiliates of the DRMCRL have unique expertise in the study of breast and prostate cancer. Our group has built critical breast and prostate cancer tissue banks and established unique preclinical models (e.g patient-derived models of disease) essential for driving the research programs forward in a clinically relevant manner. For example, we developed an ex vivo patient-derived explant (PDE) model that sustains tissue architecture, cellular complexity and hormone responsiveness of clinical samples, allowing investigation of physiological responses to hormone or drug exposure in human breast and prostate tissues (normal or cancerous). More recently, we have begun using patient-derived organoid (PDO) models on a high throughput screening platform as a validation approach prior to in vivo mouse studies with patient-derived xenograft (PDX) models.
Cell line 2D tissue culture Patient-derived explant tissue in sponge matrix Patient-derived organoids in 3D matrix suspension