A New Treatment Strategy
The DRMCRL spearheaded the collaborative program that won funding from the inaugural Movember and National Breast Cancer Foundation (MNBCF) linkage grant initiative in 2017.
The program aims to transform endocrine therapy (synonymous with hormone deprivation therapy) for breast and prostate cancer by developing ground-breaking new treatment strategies that rehabilitate, rather than completely abolish, the corrupt behaviour of ER and AR that drives breast and prostate cancers. This unique funding initiative is the first to recognise that similarities in these diseases can be leveraged to make innovative discoveries, a concept that has been the DRMCRL ethos since its inception.
View the video below to hear Professor Wayne Tilley describe the aims of the collaborative research program funded jointly by the Movember Foundation and the National Breast Cancer Foundation.
-
Our team
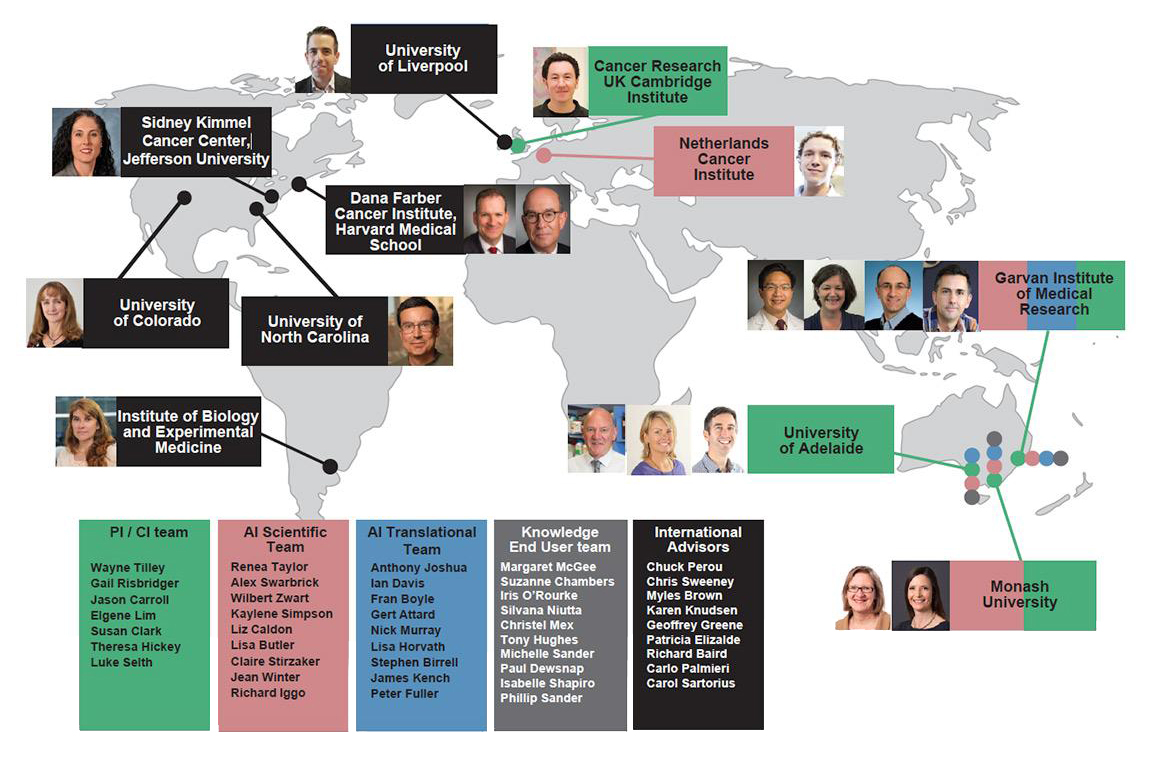
To achieve objectives of the MNBCF program, the DRMCRL convened an international, multidisciplinary collaborative team of scientific, translational, clinical and patient advocate expertise. The main body of research is being undertaken at three Australian locations: The University of Adelaide (Adelaide), The Garvan Institute for Medical Research (Sydney) and Monash University (Melbourne).
The Project Group consists of Wayne Tilley (Principle Investigator) with 6 Co-Investigators – Theresa Hickey and Luke Selth (Adelaide); Elgene Lim and Sue Clark (Sydney), Gail Risbridger (Monash) and Jason Carroll (UK).
The International and National Advisory Team include: Renea Taylor, Alex Swarbrick, and Anthony Joshua (Australia); Carlo Palmieri (UK); Paul Rennie (Canada); Chris Sweeney and Carol Sartorius (USA); Wilbert Zwart (Netherlands), and Patricia Elizalde (Argentina).
The Knowledge Translation/Advocate Team include: Christel Mex, Silvana Niutta, Paul Dewsnap, Tony Hughes, Michelle Sanders, Iris O’Rourke and Margaret McGee (South Australia) and Isabella Shapiro (New South Wales).
-
Our hypothesis
"Reprogramming estrogen or androgen receptors away from oncogenic activity will transform endocrine therapy for breast and prostate cancer."Prof Wayne Tilley, DRMCRL Director
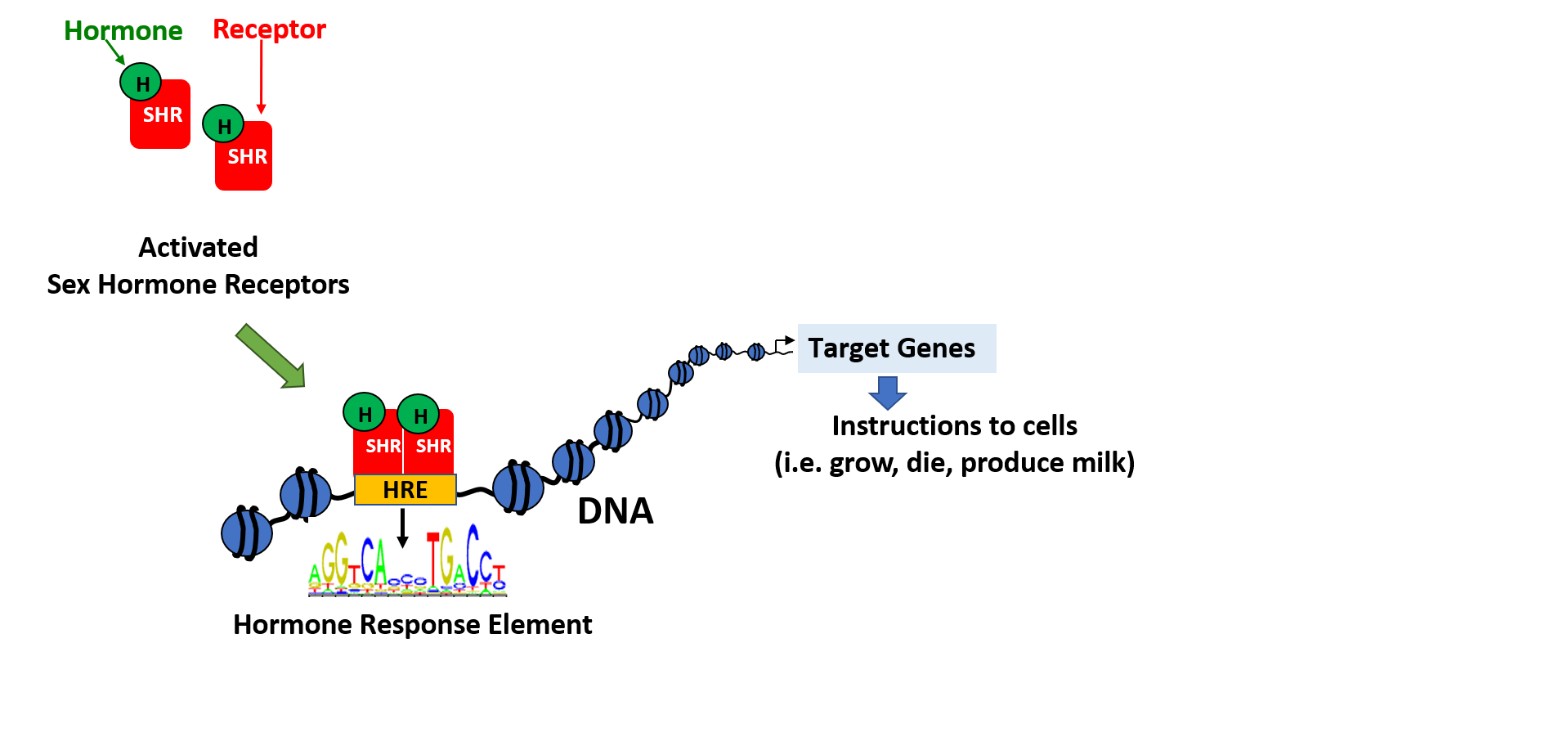
Breast and prostate cancers are diseases predominantly driven by abnormal sex hormone receptor activity. The estrogen receptor (ER) drives breast cancer and the androgen receptor (AR) drives prostate cancer. The main treatment strategy, employed for over 100 years (breast) and 70 years (prostate), is to completely abolish activity of the relevant hormone receptor in some way. This strategy is generically called endocrine therapy, but we refer to it as hormone deprivation therapy to be more descriptive.
Endocrine therapy can be highly effective for breast or prostate cancer, but is often associated with debilitating side-effects that diminish quality of life for up to five years or more while under treatment. Some patients stop taking their medication due to these side effects. Importantly, for about 30% of patients, hormone deprivation therapy does not work at all or their cancer rapidly becomes resistant; it is these therapy-resistant tumours that kill people with breast cancer or prostate cancer.
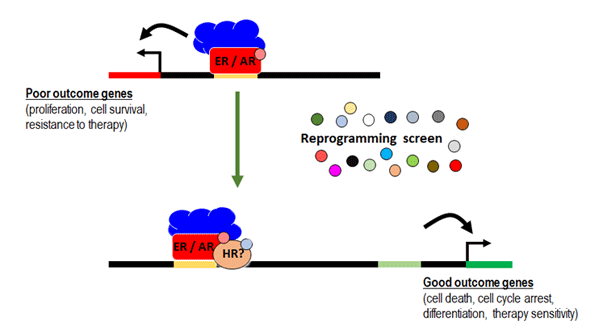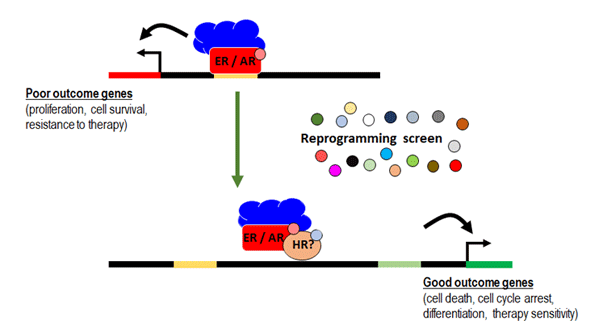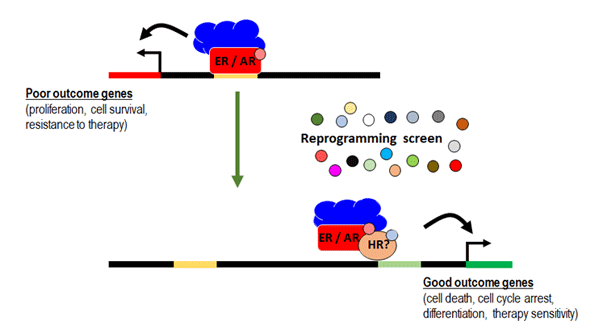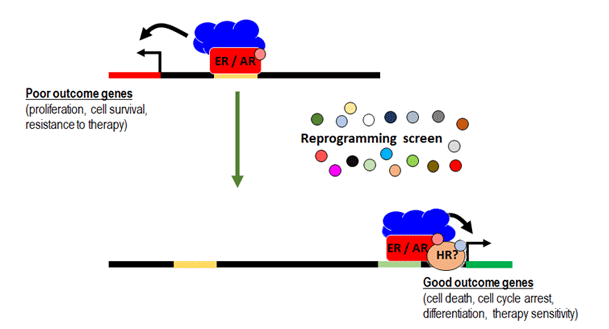
Our novel ER and AR reprogramming strategy is conceptually distinct from hormone deprivation and has potential to reduce the death rate from breast and prostate cancers by not causing the type of biological stress that promotes evolution of treatment-resistant disease. An additional benefit may be reduced toxicity and improved quality of life while under treatment, which will be evaluated in on-going or up-coming clinical trials. Our research approaches involves identification of new drug candidates, as well as the testing of repurposed drugs (i.e. drugs known to be safe and already approved for other medical purposes) for their capacity to 'push' ER and AR away from an oncogenic to a differentiated state. The latter approach will vastly increase the speed of translating findings from the laboratory to the clinic.
-
Our progress
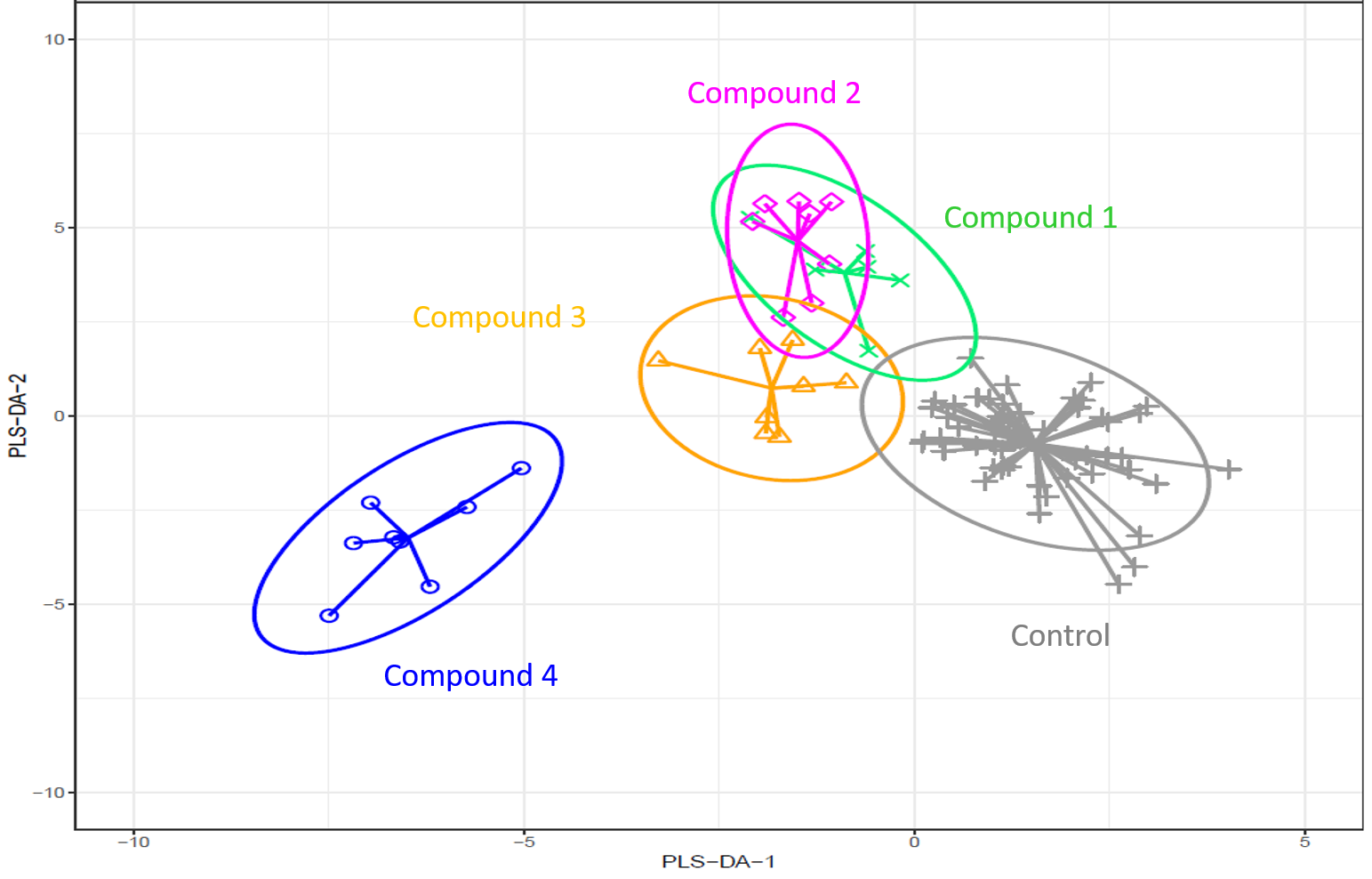
Using a panel of breast and prostate cancer cell lines, we performed a high-throughput screen of a drug library containing more than 3,200 compounds to identify candidates that inhibit cell proliferation and induce cellular differentiation. To assess cell differentiation, we developed a novel high content imaging pipeline to measure changes in cellular morphology. We then validated selected hits in a secondary screen performed in a 2D and 3D format to facilitate the ability to screen patient-derived organoids (PDOs), which requires 3D.
A series of patient-derived xenografts (PDXs) and corresponding patient-derived organoid (PDO) models of breast and prostate cancer have been generated to use as part of a novel pre-clinical pipeline to test our hypothesis. This approach is the most clinically relevant without undertaking clinical trials. Hits selected from the cell line screen mentioned above will be validated in a high-throughput screen of PDOs. The next step is to take selected candidates forward to pre-clinical testing in vivo using the PDX models.
In addition to clinical end-points, the project involves interrogating the genome, epigenome and proteome of therapy-resistant breast and prostate cancers using ChIP-seq, RNA-seq, whole genome bisulfite sequencing, and pPLEX-RIME to understand the mechanisms that regulate expression of genes that promote tumour growth and survival versus cell cycle arrest and differentiation.
A time lapse video showing breast cancer organoid growth in culture conditions.
Clustering of breast cancer organoid features after compound treatment.